
Taiwan Pharmaceutical Registration
Your TFDA Gateway
Regulatory . Certification . Licensing . Assurance
Navigate Taiwan's dynamic $2.9 billion pharmaceutical market with expert regulatory guidance. Our Taiwan-based team provides comprehensive TFDA registration services to capitalize on this import-reliant market driven by an aging population, health awareness, and rapid healthcare technology improvements.
Why Taiwan
Compelling Opportunities in Import-Dominant Healthcare Market
Strong Market Fundamentals
Taiwan's pharmaceutical market, valued at $2.9 billion in 2021, is heavily reliant on imported drugs, presenting significant opportunities for international pharmaceutical companies.
Key Market Drivers:
• Increasing social awareness of health and well-being among population
• Growing aging population driving pharmaceutical consumption
• Government efforts to promote foreign investment in healthcare sector
• Rapid improvements in healthcare technology creating demand for innovative products
Our Taiwan Services

Regulatory Challenges & Requirements
However, the regulatory landscape, including the Pharmaceutical Affairs Act, presents specific challenges requiring expert navigation:
Key Regulatory Challenges:
• Stringent compliance with Good Manufacturing Practices (GMP) requirements
• Preparation and submission of necessary documentation in local Taiwan traditional language
• Complex regulatory guidelines requiring comprehensive understanding
• Sometimes need for local clinical trials depending on product category
Documentation & Language Requirements:
• All regulatory submissions must be in Traditional Chinese
• Technical documentation requires professional translation and local compliance
• Regulatory dossiers must meet specific TFDA formatting and content requirements
• Ongoing correspondence with authorities conducted in local language
Taiwan Food and Drug Administration (TFDA) Framework
The pharmaceutical landscape in Taiwan is a dynamic and evolving sector, regulated by Taiwan's Food and Drug Administration (FDA). The FDA ensures comprehensive oversight to guarantee that:
TFDA Regulatory Standards:
• All pharmaceutical products available in Taiwan meet stringent quality standards
• Safety and efficacy requirements are rigorously evaluated and maintained
• International standards are upheld for both local and foreign pharmaceutical brands
• Confidence is instilled in pharmaceutical products through robust regulatory oversight
Regulatory Excellence & International Standards
As the FDA strives to uphold international standards, instilling confidence in both local and foreign brands, understanding the myriad of regulatory guidelines in Taiwan is vital for companies entering the pharmaceutical market.
Market Access Success Factors:
Successfully navigating these regulatory complexities is crucial for tapping into the potential of Taiwan's pharmaceutical industry. This ensures the safe and effective interplay between imported and local drugs, without compromising on quality. As such, Taiwan presents a compelling opportunity for pharmaceutical companies looking to expand their global footprint.

Ready to Enter Taiwan's $2.9 Billion Pharmaceutical Market?
Let's Navigate Your TFDA Registration Success
Don't let Taiwan's regulatory complexities delay your market entry. Our Taiwan-based regulatory experts, with deep TFDA knowledge and Traditional Chinese capabilities, are ready to accelerate your pharmaceutical registration in this compelling $2.9 billion import-reliant market.
Contact us today to discuss how our Taiwan expertise can unlock opportunities in this dynamic pharmaceutical market with strong government support for foreign investment.
Our Experiences
-
Over 15 years of experience
-
More than 20 new drugs application, including New Chemical Entities (NCE), Biological and combinations
-
Experience in the registration of generic and over-the-counter (OTC) products
Experienced in handling a wide range of product types, including New Drug Applications (NDA), vaccines, biologics, biosimilars, radiopharmaceuticals, generics, cosmetics, health supplements, and medical devices.
>14 NDA submissions in Taiwan, including NCE, Generic, and Biological products etc.
Marketing Authorization Holder (MAH) Representation as Regulatory and Local Responsible Person in Pharmacovigilance, liaison with Taiwan Food and Drug Administration, efficiently handling queries and regulatory communications for MAH approval.
Handled Marketing Authorization Transfers, regulatory support for transfer inventory and grace period between Product Owner and Distributor.
Support Plant Master File (PMF) application, obtain Company Good Distribution Practice (GDP) for MAH
Authorization of Importers
Support Tender communications with the Hospital Authority in Taiwan as Marketing Authorization Holder.
Provided comprehensive life cycle management including variations, pharmacovigilance services such as PSUR submission, ICSR, product defect handling, etc.
Delivered regulatory intelligence reports, dossier gap analysis to facilitate successful market entry.
Handled Product Owner regulatory and Pharmacovigilance Audits

Our Establishment
To become a Pharmaceutical Marketing Authorization Holder (MAH) or Product Registration Holder in Taiwan, you need to follow these steps:
1. Company Registration: Register your company in Taiwan with the relevant authorities.
2. Pharmaceutical License: Apply for a pharmaceutical license from Taiwan's Food and Drug Administration (TFDA).
3. Good Distribution Practice (GDP): Ensure your distribution practices comply with GDP guidelines.
4. PMF and DMF Registration: Register and host your Product Master File (PMF) and Drug Master File (DMF).
5. Product Registration: Register your pharmaceutical products with the TFDA.
6. Authority’s Platform Access: Gain access to the TFDA’s platform for regulatory submissions and updates
You need a trustworthy and experienced professional consulting firm that offers comprehensive services to help handle complex and tedious matters. PharmEng, providing end-to-end support, is your best choice.
.jpg)
Overview of Pharmaceutical Product Registration in Taiwan
Taiwan Food and Drug Administration(TFDA), under the Ministry of Health and Welfare((MOHA), is the authority responsible for evaluating and approving pharmaceutical products, Medical Devices, Cosmetics and Food, Inspection, Post Marketing Surveillance, Science & Research. The process involves multiple steps to ensure that products meet stringent safety, quality, and efficacy standards.
Medicinal Product Classification in Taiwan
Medicinal products are classified into the following categories:
Review of Other Categories by the TFDA
Registration Requirements for Pharmaceutical Products in Taiwan
International
• WHO, ICH, EMA, US-FDA
Local
• Regulations for Registration of Medicinal Products
The pharmaceutical product registration process in Taiwan involves the following key steps:
01
Determine Product Classification
Proper classification of the products and their review process, such as New Drug Application (NDA), Abbreviated New Drug Application(ANDA), Non-Prescription Drug Registration
02
Appoint an Authorized Representative
Foreign manufacturers must appoint a local authorized representative to liaise with Taiwan Food and Drug Administration (TFDA) and The Center of Drug Evaluation(CDE).
03
Submit Application via ExPress
Applications must be submitted electronically through online platform (ExPress)
04
Dossier Submission
CTD format registration dossier, including comprehensive data on product formulation, manufacturing processes, stability studies, clinical trial data, and labeling etc.
05
Evaluation and Review
The CDE evaluates the dossier for compliance with safety, quality, and efficacy standards, and access the benefit and risk for use in Taiwan. Additional documents or clarifications may be requested during this phase.
06
Approval and License Collection
Upon successful evaluation, a product license is issued, allowing the product to be marketed in Taiwan.
Example of Flowchart* for NDA Application and Review
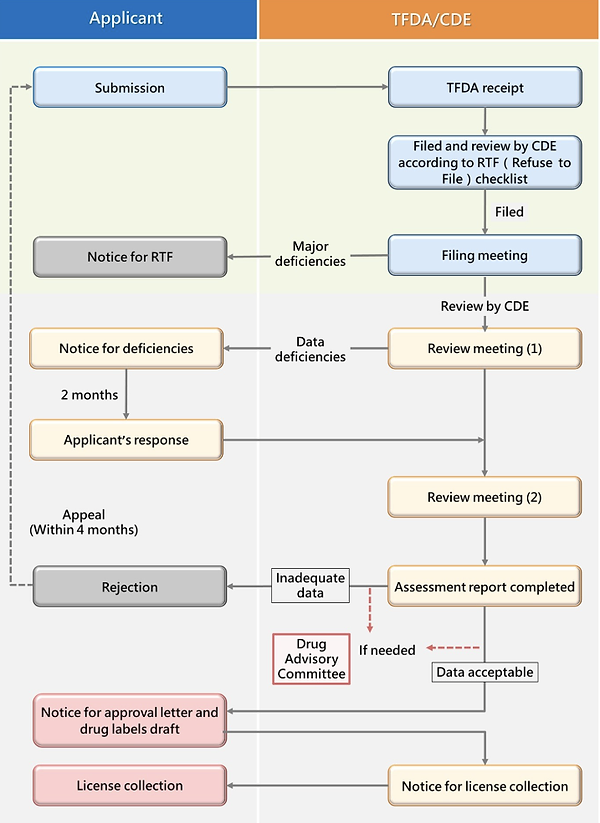
*Data sourced from the CDE website: New Drug Application (NDA)-Drugs
Authority Registration Timeline for Pharmaceutical Products in Taiwan
Application Type | Application Category | Timeline (Working Days) |
|---|---|---|
New Drugs | New Chemical Entities (NCE)
| 360 |
Biologics | ||
Rare disease | ||
Other New Drugs | New ttherapeutic compounds | 300 |
New administration route | ||
New dosage form, new dose unit, new unit strength | ||
Generic Drugs | Generic drug | 180 |
OTC products |
*Refer to the TFDA official website 案件辦理期限公告 - 查驗登記專區 - 藥品 - 業務專區 - 衛生福利部食品藥物管理署.
Authority Registration Fees for Pharmaceutical Products in Taiwan
Application Type | Application Category | Review Fee (TWD) |
|---|---|---|
New Drugs | New Chemical Entities (NCE) | 1500,000 |
New therapeutic compound or new administration route | 500,000 | |
New dosage form, new dose unit, new unit strength or controlled release forms, new strength of the same therapeutic compound(s) and the same administration route | 250,000 | |
Biological Drugs | Blood product, anti-toxin, or vaccine | 1500,000 |
Gene-engineering | ||
New dose package or new manufacturing site | 250,000 | |
Generic Drugs | Generic Drugs-under pharmacovigilance | 140,000 |
Generic Drugs-not under pharmacovigilance | 80,000 | |
Plant Master File (PMF) | A PMF | 120,000 |
An additional dosage form | 20,000 |
*Refer to the official website Fee-charging Standards for Reviewing the Registration of Western Medicines for detailed and the latest information.
How can we help?
• Pre-Market Strategic Consulting and Feasibility Assessment
• Regulatory intelligence and market entry strategy
• Product classification and regulatory gap analysis
• GMP compliance auditing and advisory
• Application for Plant Master File (PMF) or Support for overseas inspections
• Dossier preparation and submission handling
• Local Entity Support and Good Distribution Practice (GDP) approval
• Life Cycle Management such as post-market surveillance and pharmacovigilance support
• Pre-submission consultation activity with Health Agency
• All types of Import License Application
• Change of Product Registration Holder
• Ad-hoc Regulatory Affairs Consultation
















